NetraMark and Worldwide Clinical Trials Collaboration to Transform Clinical Trials
NetraMark and Worldwide Clinical Trials Collaboration to Transform Clinical Trials
.png)
NetraMark and Worldwide Clinical Trials have partnered to integrate an AI platform, "NetraAI," across neuroscience and oncology clinical trials. At a pivotal time when inefficiencies are dragging down drug development timelines, this initiative aims to address some of the biggest obstacles for the pharmaceutical industry.
Harnessing AI to Tackle Complexity in Clinical Trials
AI's integration into clinical research is changing the very make-up of clinical trials and how they are carried out. Traditionally, they are limited by static methodologies and inefficiencies, but are beginning to evolve dynamically with the support of data-driven technologies.
This is particularly relevant in therapeutic areas such as oncology and neuroscience, where complex disease mechanisms, patient heterogeneity, and large volumes of data present ongoing challenges to trial design and execution.
As healthcare organizations seek innovative ways to accelerate timelines and improve outcomes, AI is stepping in—not as a replacement for scientific expertise, but as a powerful partner.
A recent partnership between NetraMark and Worldwide Clinical Trials exemplifies this shift, offering a glimpse into how machine learning and deep data analytics can be harnessed to make clinical trials more precise, patient-centric, and ultimately more successful.
Longstanding Challenges in Clinical Research
Clinical trials today face a set of deep-rooted challenges:
- Low Patient Recruitment and High Dropout Rates. Nearly 80% of clinical trials struggle to meet their enrolment timelines, and one in five fail to recruit a single participant. Recruitment challenges are further amplified in certain therapeutic areas.
In neuroscience, stigma surrounding neurological conditions can discourage patients from participating. In oncology, strict eligibility criteria combined with the physical and emotional toll of treatments make both recruitment and retention particularly difficult.
- Trial Complexity. Modern trial designs often target specific populations or look at multiple endpoints, making trial execution more difficult and expensive. Trial design aside, developing effective treatments is challenging in the first place, in part due to the intricate nature of the human body and the underlying mechanisms of diseases.
In oncology, pinpointing reliable biomarkers is still a major challenge—tumors are incredibly complex ecosystems with up to 99% of their genes mutated. This means that, even if two patients have the same cancer type with some overlapping biomarkers, their molecular profiles likely differ substantially—in some cases, tumors of the same type have no overlapping mutations.It’s like looking at two forests from a bird’s-eye view: they might look the same at first glance, but up close, they have entirely different species, undergrowth, and soil.Because of this heterogeneity, developing and validating treatments is incredibly difficult.
These nuances also translate to neuroscience—brain disorders are incredibly intricate and often poorly understood. For example, one study showed a mutation associated with autism spectrum disorder in some individuals could manifest as borderline personality disorder in others—even if they belonged to the same family.
This points to a strong underlying issue: developing treatments that work for all patients across one disease is almost impossible.
- Data Overload and Inefficiencies in Analysis. With huge volumes of patient data from electronic health records (EHRs), wearables, and biomarkers, synthesizing this data for actionable insights is a growing challenge.
- Regulatory Delays. Regulatory approval is tightly linked to the robustness and clarity of trial results, which are often compromised by insufficient study design. Tying in with trial complexity, scientists often won't know the "ideal" patient population for a drug until it reaches a late stage of development, dragging out the regulatory process.
A Partnership Built on AI Precision
Earlier this month, NetraMark Holdings Inc., a leading AI company specializing in precision analytics for the pharmaceutical industry, announced a global agreement with Worldwide Clinical Trials, a full-service contract research organization. This strategic partnership aims to integrate NetraMark's new NetraAI platform into Worldwide's service offerings, initially focusing on Phase 2 neuroscience and oncology clinical trials, with plans to expand across all therapeutic areas and trial phases.
The NetraAI platform is designed to uncover hidden patient subpopulations within complex datasets, enabling more precise patient stratification and trial optimization. By leveraging this technology, the partnership seeks to enhance trial efficiency, reduce costs, and improve the likelihood of regulatory success.
Josh Spiegel, chartered financial analyst and president of NetraMark, expressed enthusiasm about the collaboration, stating:
"This partnership represents a significant step forward in our mission to revolutionize clinical trials through AI. By combining our innovative technology with Worldwide's extensive experience, we aim to bring unprecedented efficiency and precision to the drug development process."
Peter Benton, CEO of Worldwide Clinical Trials, echoed this sentiment, noting, "Integrating NetraMark's AI capabilities into our clinical trial processes aligns with our commitment to delivering cutting-edge solutions for our clients. This collaboration is poised to enhance our ability to design and execute trials that are both efficient and patient-centric."
Translating Jargon into Real-World Impact
But with so many “innovative” AI technologies promising to transform the industry, how does NetraAI slot in?
The answer lies in NetraMark’s research. In February 2025, the company published research outlining a novel AI method to predict “good” or “bad” responses to antidepressants. While researchers have long used machine learning to uncover patterns for predicting treatment outcomes, denoising these patterns is a challenge—even AI struggles to understand what characteristics directly cause an outcome, and which ones are merely associated.
For instance, machine-learning analysis of a big dataset might reveal that white, unmarried males are likely to respond worse to an antidepressant than the rest of the population. While race and sex might directly influence a drug’s effect, being “unmarried” will not—this is just a correlated trait. Traditional AI algorithms can’t “see” this difference between cause and correlation, making it difficult to truly understand what characteristics are important for predictions.
With their novel approach, “sub-insight learning,” NetraMark aimed to bridge this gap, building a pipeline with four key features:
- A “dynamical system,” that looks at how things change in sequence (i.e., how 'A' leads to 'B,' which leads to 'C'), rather than just capturing snapshots of patient characteristics (i.e., 'A' and 'B' happen at some point in time).
- Attention mechanisms, which let the model focus on the most important features for predictions and cut out the irrelevant data.
- Semi-supervised learning. The model is trained on both unlabeled (where the outcome is unknown) and labeled (where the outcome is known) data.This combines the best of both worlds from supervised and unsupervised learning: providing the model with labeled data points out the crucial patterns that cannot be ignored, while the unlabeled data helps uncover more subtle links that might be missed otherwise.
- Multiclass classification. Instead of classifying patients into two groups (responders or non-responders), the model also groups patients into an “unknown” group.Here, if a patient responds well or poorly to the treatment, and the model understands why, they are sorted into “good” or “bad” response groups, respectively.But, if the AI can’t explain their outcome, the patient is assigned the “unknown” label and they are excluded from the training dataset—essentially, this tells the model: “If the pattern doesn't make sense, don't guess. Just admit you don't know.”
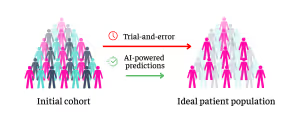
To see if their new method could make a difference to AI performance, the researchers added these new features onto the "building blocks" of traditional machine learning models—refining pre-existing frameworks rather than starting from scratch.
And the results were telling. On average, accuracy improved by 28%, with one model’s score increasing from 60% to 100% after applying sub-insight learning. Sensitivity—the ability to correctly identify the “responders”—rose by ~31% while its counterpart, specificity—the ability to correctly identify the “non-responders”—increased by over 50%.
More tangibly, this meant that, armed with a new “toolkit,” the AI models could better predict which patients were likely to get on well with a treatment, and which ones would not.
It’s this initiative that the NetraMark-Worldwide collaboration tunes into. In a clinical trial setting, deploying novel AI pipelines could quickly pinpoint a new treatment’s ideal patient population without spending years on “trial-and-error" experimentation—not only sparing millions in costs, but more importantly, reducing risks to human lives.
Unlocking the Future of Clinical Trials with AI
More broadly speaking, AI is transforming the clinical research landscape, making trials faster, smarter, and more tailored to patient needs. By addressing long-standing challenges such as recruitment hurdles, data overload, and complex protocol designs, AI offers a powerful path forward—especially in fields like neuroscience and oncology where traditional methods often fall short.
Key Benefits of AI Integration in Clinical Trials
- Smarter Patient Recruitment and Retention. AI can identify specific patient subpopulations by analyzing patterns across clinical, genomic, and behavioral data. This enables more targeted recruitment strategies, reduces enrollment times, and improves retention, particularly important in trials where stigma or treatment intensity are barriers to participation.
- Simplified and Adaptive Trial Designs. Complex trials, common in neuroscience and oncology, often involve diverse patient populations and multiple endpoints. AI supports the development of adaptive, streamlined protocols that can accommodate this complexity.
- Real-Time Data Monitoring and Insights. With access to biological data growing almost exponentially, AI helps researchers make sense of it all, not only analyzing a lot of data, but in real-time—when it matters most. Researchers can monitor patient responses and safety signals as they unfold, allowing for timely adjustments throughout the trial.
- Advancement of Personalized Medicine. By uncovering novel therapeutic targets, AI helps develop more effective, personalized treatments. This is critical in areas like oncology and neuroscience—even if two patients technically "look" the same on paper, one individual's disease might have a completely different genetic landscape from the other's.
- Improved Regulatory Alignment. AI’s ability to generate robust, high-quality data and clear insights into treatment responses supports the development of trial protocols that align more closely with regulatory requirements, helping pave the way for faster, smoother approvals.
- Reduced Costs and Time to Market. According to recent studies, AI-enhanced clinical trials can reduce costs by up to 30% while shortening development timelines by over 20%.
Implications for the Future
The NetraMark–Worldwide collaboration is indicative of a broader trend, marking AI's transition from proof-of-concept to operationalization in healthcare. As pharma companies face increased pressure to deliver safe and effective therapies faster, partnerships like this offer a blueprint for modern, data-driven clinical development.
With the power of machine learning, subpopulation detection, and personalized analytics, the collaboration promises not only cost savings and faster approvals but also improved treatment outcomes for patients worldwide.
.png)
.png)
.png)